

Other Black Holes may have formed during the first seconds of the Big Bang. In some respects, Black Holes are an approximation to the supersingularity postulated as the starting point of the Big Bang except that they have finite dimensions of meters to several kilometers and even much larger for those in galactic centers depending on their amounts of mass (can be equivalent to the cumulate mass of hundreds of millions to billions of Suns). Speculatively, one future outcome for the Universe, after 50 b.y. or so, could be a collection of billions of Black Holes that eventually converge upon themselves to coalesce into a single ultra-dense Black Hole that ultimately would become the singularity for the next Universe (in this model, any number of successive Universes, exploding and contracting cyclically, is feasible). One view in quantum theory holds that Black Holes can nevertheless emit particles/radiation (Hawking radiation) and are thus capable of disappearing or "evaporating" through radiative dissipation.
Stars, particularly the massive ones, are the furnaces in which the elements beyond H, He, and some Li are synthesized by successive steps in nuclear fusion in which more and more protons and neutrons are joined into stable to unstable nuclei. A typical, but somewhat generalized sequence of nucleosynthesis is depicted in the figure below for a star composed initially of 20 solar masses (MO) of hydrogen, but now in its final stage of evolution in which the star consists a sequence of elements formed progressively with depth as it heated up and contracted. The relative proportions of each element are given by the numbers in each shell. Stars with greater than 10 solar masses will proceed to the iron core stage; a Sun-sized star reaches only the carbon core stage.
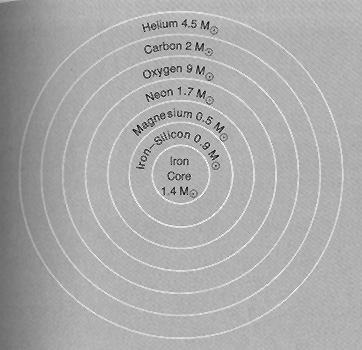
From J. Silk, The Big Bang, 2nd Ed., © 1989. Reproduced by permission
of W.H. Freeman Co., New York
As a massive hydrogen-rich star contracts and experiences greater pressures, helium is the first nuclear product within its core region. The energy released from fusion, along with continuing densification, yields higher temperatures (~ 108 °K) that transmute this innermost helium into carbon while producing new helium at the next outer shell, but with hydrogen still dominant. Once carbon is formed in abundance, this helium is generated as an end product of the CNO cycle. In this, some C12 reacts with protons to generate, in successive steps, nitrogen 13, 14, and 15 and then oxygen 15. After this last step, that unstable oxygen isotope can decay by fission, thereby releasing an alpha particle (He4, stripped of its electrons) causing the reversion to C12.
Ever greater contraction, with concomitant temperature rises (> 1010 °K) , can progressively generate the elements listed in the figure up to iron (plus others of lesser atomic numbers) in amounts proportional to the comparative solar masses indicated. Thus, a star massive enough to ultimately achieve an iron core also contains elements of lower atomic numbers in its outer shells, broadly distributed in the relative positions shown in the figure, reflecting response to the fuel to outwardly decreasing densities and temperatures. Iron (atomic number Z [no. of protons]= 26; mass number A [number of protons + neutrons] = 56) is the heaviest element producible directly by stellar fusion. In fusion, nuclear binding energies for the new nuclides increase gradually up to iron but the mass of a fused nuclide is less than the sum of the fusing constituents; the missing mass is converted to energetic particles (E = mc2), released as gamma rays, neutrinos, positrons, and others.
Elements with A greater than Fe have decreasing binding energy and to form require energy input from non-fusion processes (principally neutron capture). Because those stars capable of synthesizing elements up to Fe have masses greater than 10 solar masses, these stars at their end stage of fusion will rapidly (over spans of hundreds of years) collapse and explode (fly apart) as novae or supernovae. This gives rise to intense neutron fluxes that manufacture various elements including those with A > 56 , most of which become rapidly dispersed into interstellar space. These heavier elements, along with H, He and the A < 56 elements (which include O, S, C, N, Fe, Mg, Ca, Al, Na, and K - the dominant constituents making up the planets), can thereafter collect into new nebulae (clouds) that may reorganize into additional stars, setting up further nucleosynthesis. The elements were mostly created in the first few billion years when rates of star formation, burning, and explosive destruction were higher than present, but the process of element production still goes on. Elemental materials not reincorporated in stars are available to organize into compounds that make up the dust, gases, and particles from which planetary bodies are assembled.

Collaborators: Code 935
NASA GSFC, GST, USAF
Academy
Contributor Information
Last Updated: September '99
Webmaster: Bill Dickinson Jr.
Site Curator: Nannette Fekete
Please direct any comments to rstweb@gst.com.